Since the first confirmed COVID-19 case reported in the United States in late January, the nation has seen more than 1.6 million confirmed cases and approximately 96 thousand deaths. This number has risen higher than anticipated while states have been staying under lock-down orders for months. As state and federal governments plan for reopening the economy, promising vaccine candidates are moving into their second clinical trials. This timing pushes the hopeful availability of vaccine for mass production to later this year or next year. With uncertainty around a vaccine, testing capacity may become the next key strategy to ease social distancing and reopen the parts of the country that are still under lock-down.
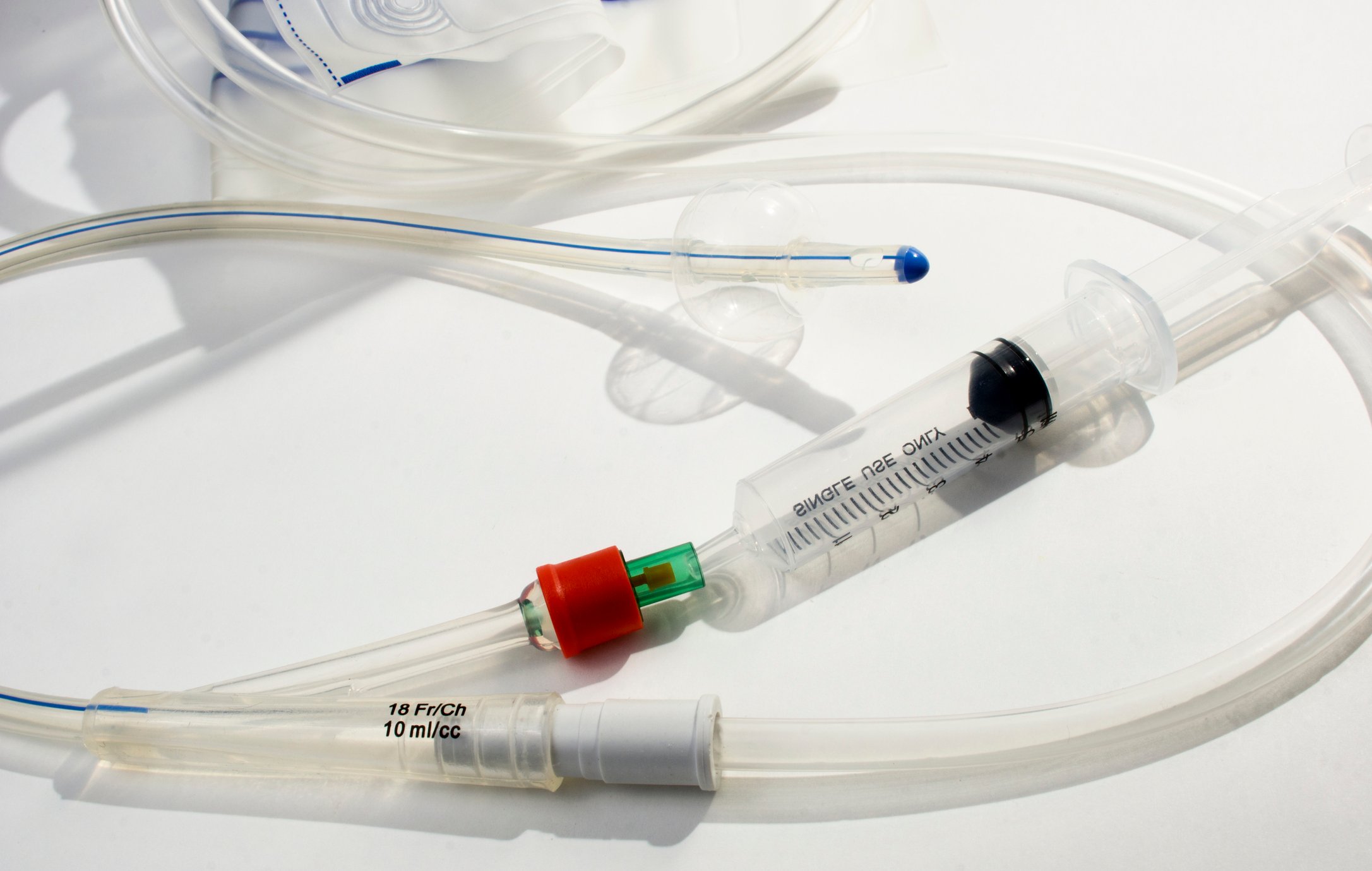
So why has it been difficult to produce swabs and keep up with demand? They’re not simple Q-tip style swabs that you would find at a local supermarket. The kind you can get in store have cotton tips that contain DNA material and could interfere with the detection of the viral genetic material in the lab. Nasopharyngeal swabs are specialized medical devices that are flexible and long enough to travel through a person’s nose to collect specimen at the nasopharynx, which is at the base of the skull and above the roof of the mouth. Late last month, the government awarded a $75.5 million contract to Puritan Medical Products to produce more swabs. Many other companies are also ramping up production of medical grade swabs, including some 3D printed swabs with the innovative lattice design to collect specimens.
Of course, a strategy with nasal tests as part of our new normal would require very frequent tests for individuals, as you may come in contact with the virus after a negative test or could be asymptomatic for weeks. While an antibody test offers a more permanent sense of security, its accuracy is questionable. Furthermore, the strategy of social distancing was to ensure low exposure and infection rate, and not to achieve herd immunity.
There are also a few alternatives to individual nasal swabs and blood tests. A different site of specimen collection such as an oral swab, a saliva test, or a different format like a group test that would reduce the overall number of swabs needed. Home test kits are also becoming more readily available as well (see this article for another PackTalk post on home collection kits approved by the FDA).
As public establishments are slowly opening throughout the country and certain industries are opening their doors for employees to return, employers are investing more into safety precautions, screening and social distancing measures. Frequent and efficient testing could be another key asset for prevention of another wave of cases, while waiting for an effective a vaccine to be developed and manufactured. It is noteworthy that early and aggressive testing is a strategy employed by many countries such as Germany, South Korea, Iceland, and Vietnam. More information on these approaches and how these strategies impact the packaging world here.
At Oliver, we are committed to contributing in the fight against COVID-19. Our stock pouch program comes with a promise of high-quality and next day shipping options for any swab packaging needs.