Flu Fighters: A Look at Pharmaceutical Packaging
As the world continues to grapple with the ongoing challenges of infectious diseases, National Influenza Week (observed in the US) serves as a vital global reminder of the persistent threat posed by seasonal flu. This article aims to explore the vaccine industry and the infrastructure, regulation and environmental conditions that influence the industry in both APAC and the Americas regions.
What kind of pharmaceutical packaging infrastructure exists in both regions today?
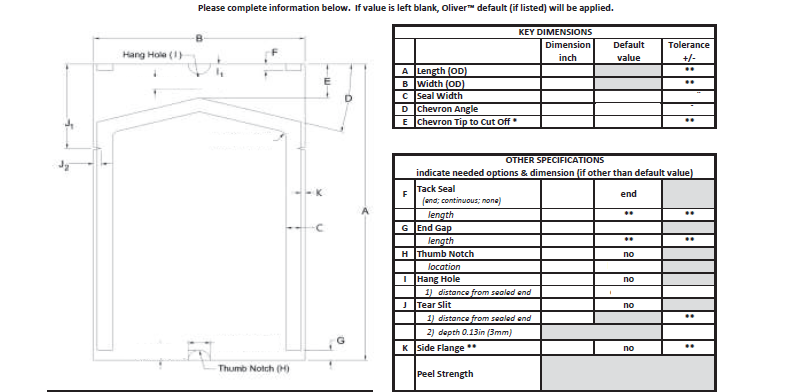
The pharmaceutical infrastructure varies greatly depending on the region and the overall development of the country. The tables below summarize the infrastructure trends we observe in APAC and Americas respectively:
|
APAC Pharmaceutical Infrastructure |
|
|
Developed Countries |
Developing Countries |
|
|
|
Americas Pharmaceutical Infrastructure |
|
|
Developed Countries |
Developing Countries |
|
|
From the tables above, it is important to note that developing pharmaceutical infrastructure helps scale up supply during mass immunization programs or pandemics. In comparison, developing countries often have a slower ramp-up in supply as they have to wait for imports before rolling out any mass immunization efforts.
Is the pharmaceutical packaging regulatory climate different amongst APAC and Americas?
Regulatory climate differs extensively amongst developed and developing countries in APAC and Americas. The table below lists the regulatory climates affecting APAC and the Americas respectively:
|
APAC Regulatory Climate |
|
|
Developed Countries |
Developing Countries |
|
|
|
Americas Regulatory Climate |
|
|
Developed Countries |
Developing Countries |
|
|
Based on the information above, one important consideration for pharmaceutical companies is regulatory approval times. In developed countries, approval times are generally quicker as many pharmaceutical companies are familiar with regulations in developed countries. Comparatively, approval processes may be lengthier in developing countries due to a lack of clarity or specificity in their submission processes.
How do environmental conditions affect pharmaceutical packaging in developed and developing countries?
APAC & Americas
Countries across both the APAC region and the Americas face diverse climate extremes, with tropical conditions and high humidity near the equator, and more seasonal fluctuations in northern and southern hemispheres. Elevated temperatures can compromise vaccine stability, while colder climates may affect the durability of packaging materials. In developing countries, many companies are facing a lack of cold chain infrastructure. This results in a significant gap in the last-mile distribution, which may affect the products adversely. In developed countries, companies mitigate these challenges by using temperature and brittle-resistant vials and ampoules. This helps ensure vaccines remain effective across a broad range of environmental conditions from production to end-use.
The fight against seasonal flu and infectious diseases relies heavily on effective pharmaceutical packaging. While developed countries benefit from advanced infrastructure, comprehensive regulations, and streamlined regulatory processes, developing countries face challenges such as slower approval times and limited cold chain capabilities. Environmental factors like extreme temperatures further complicate vaccine stability and packaging requirements. Looking forward, strengthening infrastructure, improving regulations, and enhancing cold chain systems will be essential for ensuring timely and effective vaccine distribution globally. This is particularly relevant for regions with less developed healthcare systems.